
The concentration of dissolved hydroxide ions is twice that: So the concentration of the dissolved magnesium ions is the same as the dissolved magnesium hydroxide: Calculate the solubility product.įor every mole of magnesium hydroxide that dissolves, you will get one mole of magnesium ions, but twice that number of hydroxide ions. The solubility of magnesium hydroxide at 298 K is 1.71 x 10 -4 mol dm -3. This next example shows you how to cope if the ratio is different.

These calculations are very simple if you have a compound in which the numbers of positive and negative ions are 1 : 1. People often try to enter x 10 in the middle of this process as well. To enter this number, you would enter 1.05, press the EXP button, and then enter -5 (probably by entering 5 and then pressing the +/- button). If you try this sum, and get a different answer, then you are probably misusing the EXP button.
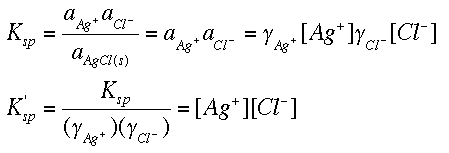
Important: Get your calculator and work this out! Students frequently mis-enter numbers like 1.05 x 10 -5. Notice that each mole of barium sulphate dissolves to give 1 mole of barium ions and 1 mole of sulphate ions in solution.Īll you need to do now is to put these values into the solubility product expression, and do the simple sum. The solubility of barium sulphate at 298 K is 1.05 x 10 -5 mol dm -3. If it was in g dm -3, or any other concentration units, you would first have to convert it into mol dm -3. I am going to assume that you are given the solubility of an ionic compound in mol dm -3. These are covered in more detail in my chemistry calculations book.Ĭalculating solubility products from solubilities This page is a brief introduction to solubility product calculations.


 0 kommentar(er)
0 kommentar(er)
